Research Summary
The ultimate goal of the Dodd laboratory is to translate insights gained from primary mouse models into findings that will benefit patients. Our research program designs and utilizes powerful in vivo model systems to directly address critical questions in tumor biology. Specific areas of research are shown below.
Genetics of Sarcoma
Genetics of Sarcoma
Genetics of sarcoma
Our lab has significant experience developing mouse models of sarcoma. We have created a novel mouse model of malignant peripheral nerve sheath tumor (MPNST) to serve as a preclinical platform for combination therapy (Dodd et al, 2013). Using the Cre-loxP system, sarcomas develop through deletion of neurofibromin (NF1), a negative regulator of Ras. Mutations in the NF1 gene have been recently identified in a wide spectrum of cancers, including lung, brain, breast and spontaneous sarcomas. Additionally, neurofibromin plays a central role in the cancer predisposition syndrome Neurofibromatosis Type I (also abbreviated NF1), an extremely common genetic condition that affects 1 in 3,000 children. The unique genetics of Neurofibromatosis Type 1 make this an elegant model to study the contribution of clinically-relevant changes in the tumor microenvironment using a genetically tractable system.
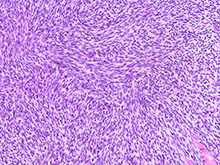
MPNST generated in Cre/loxP mice
Tumor microenvironment TME
Tumor microenvironment TME
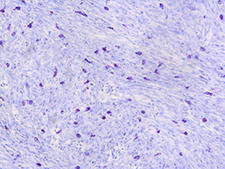
Mast cells infiltrate tumor tissue
The tumor microenvironment plays an important role in cancer biology. We designed a unique mouse that uses Cre-loxP technology to reflect the distinct genetics of stromal cells found in patients with Neurofibromatosis Type 1. We are currently using these models to define the contribution of distinct TME cell populations to sarcoma biology. We have determined that NF1 haploinsufficiency in the tumor microenvironment accelerates tumor onset and increases immune cell recruitment to the tumor. Ongoing investigations are characterizing the myeloid cell infiltrate in these tumors as a potential therapeutic target. These mice are also the subject of a preclinical trial examining how the tumor microenvironment influences response to multi-agent chemotherapy.
We are using our models to investigate promising treatments for sarcoma patients. By generating sarcomas through through injection of an Adenovirus expressing Cre recombinase, we can control the timing and location of primary tumors. This facilitates preclinical applications by allowing tumors to be closely monitored during treatment. In addition, the tumors are surrounded by a native tumor microenvironment and an intact immune system, which allows us to study the impact of tumor stroma on therapeutic outcomes. We have utilized these models as a preclinical platform to investigate molecularly-targeted therapy, novel drug delivery vehicles, and chemotherapy regimens.
Generation of Mouse Models
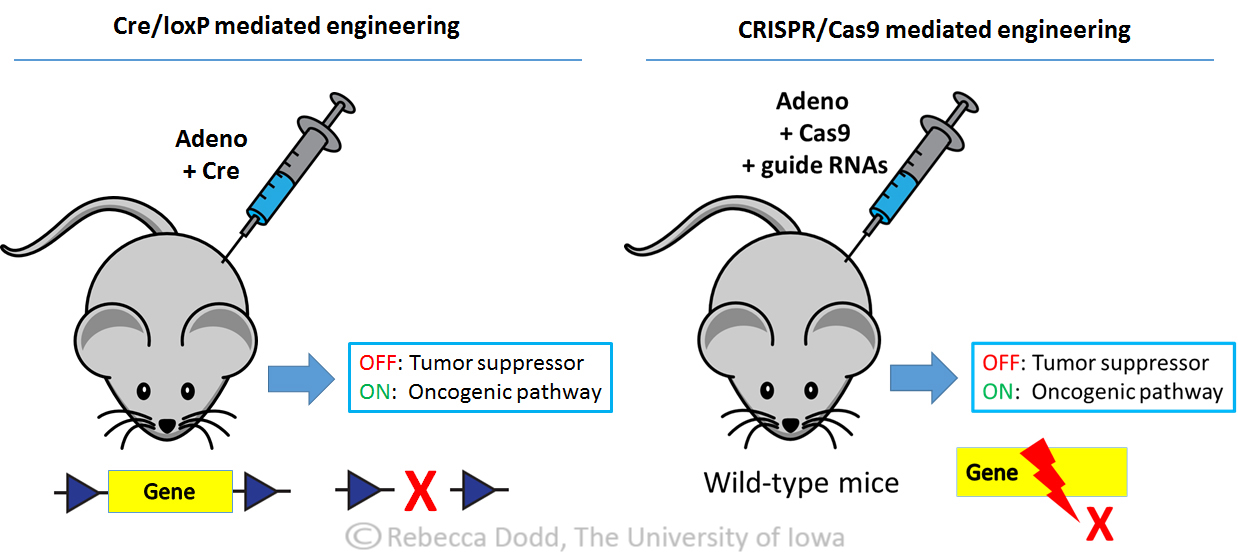
Novel genome editing tools – Cre/loxP and CRISPR/Cas9
Novel genome editing tools – Cre/loxP and CRISPR/Cas9
Mouse models of cancer are invaluable tools for understanding tumor biology and therapeutic response, although highly sophisticated Cre/loxP models require significant time and expense. New CRISPR/Cas9 technology allows cancer biologists to model tumor mutations in unprecedented ways. Through delivery of Cas9 endonuclease and target-specific guide RNAs, CRISPR/Cas9 generates indels (insertions and/or deletions) to create defined loss-of-function mutations in adult animals to study tumor formation. This technology allows for rapid screening of multiple gene mutations in mice with a fraction of the cost and time required for genetically-engineered models.